TiO2 (Titanium Dioxide)
Titanium dioxide is a titanium oxide with the formula TiO2. A naturally occurring oxide sourced from ilmenite, rutile and anatase, it has a wide range of applications. It has a role as a food colouring. Titanium dioxide, also known as titanium(IV) oxide or titania, is the naturally occurring oxide of titanium. It is used as a pigment under the names titanium white, Pigment White 6 (PW6), or CI 77891. It is typically extracted from ilmenite, rutile and anatase.
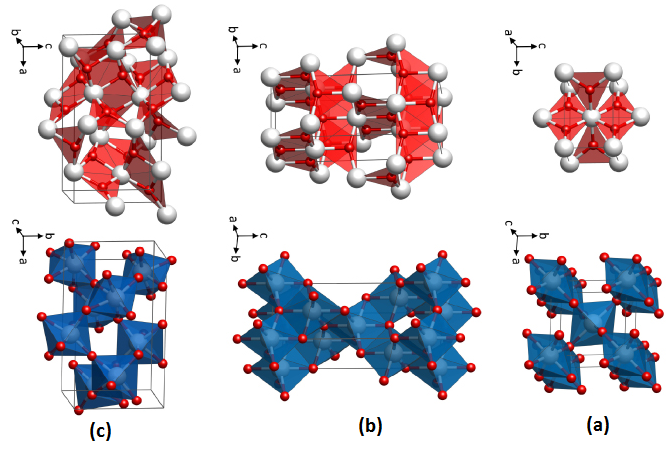
Titanium dioxide is found in nature in three different forms: rutile, anatase and brocade. Rutile is the most abundant and stable mineral, containing 98% TiO2, and is used in the production of high-temperature dielectrics and oxygen sensors. Anatase and broccoli are converted to rutile by heat. Anatase is a semiconductor material that is mainly used in optical coatings, light-sensitive paints and optical catalysts due to its low cost and strong oxidizing power against UV rays and its hydrophilic nature. Spatially, the rutile and anatase phases are tetragonal, and the bronchitis is orthomobic. Figure 1 shows the structural phases of Anatase, Rutile and Brookite.
Fig. 1) The structural phases of Anatase, Rutile and Brookite
Rutile
In a tetragonal structure, with 6 atoms per unit cell, rutile is the most stable having TiO2 octahedron showing a slight orthorhombic distortion. Rutile phase is stable while at these conditions TiO2 converts thermodynamically into auspicious phase.
Anatase
TiO2 octahedron distortion is significantly large for anatase phase while TiO2 has a tetragonal structure, hence, the symmetry of anatase is lower than orthorhombic. The energy change between these 2 phases is minor closely (∼2 to 10 kJ mol−1). At 0K, the rutile phase is not more thermodynamically constant than anatase.
Brookite
It is one of the 3 naturally occurring polymorphs of TiO2, which belongs to orthorhombic crystal system. It contains a larger cell volume with 8 TiO2 groups per unit cell. Brookite is also the least dense form compared to anatase and rutile.
Titanium dioxide micro- and nanoparticles have been synthesized via hydrolysis, sol-gel, micro-emulsion or reverse micelles, flame pyrolysis, solvo/hydrothermal, chemical vapour deposition and physical vapour deposition. Among these techniques hydrolysis is preferable because its operating conditions are the closest toambient temperature and pressure. Anatase and rutile are commonly synthesized by hydrolysis of titanium compounds such as titanium tetrachloride, titanium sulfate or titanium alkoxides in water solution. Producing the pure brookite phase is a difficult process, so most of the studies have reported the synthesis
process for the mixture of brookite, rutile and/or anatase phases. TiO2 (B) was prepared by hydrolysis of K2Ti4O9 followed by heating at 500°C.
Alkoxide is another precursor which has been used for producing different phases of TiO2 in aqueous solutions of variety acids and bases. Metalalkoxide is usually used as a precursor in the sol-gel method, which produces fine, spherically shaped powders with uniform size <1.0 mm. The high price of the alkoxide has limited the commercialization of this method.
Application of titanium dioxide
Pigment
The use of this substance as a pigment in the form of anatase was first reported in 1923 in France. Today, titanium dioxide is the most important pigment or white pigment that, in addition to staining particles, also has a protective effect against UV.
Solar Cells
In solar cells, this substance can be used as a substrate for DSSC cells due to its small particles and sunlight.
Catalysts
Due to the small particle size and high surface area of this material, TiO2 as a nanomaterial allows various catalysts to be produced with high surface activity. Recently, a lot of research has been done on TiO2 due to its high level and stabilization of catalysts in the mesopuric structure as an alternative to heterogeneous catalysts.