Influence of Dimethyl disulfide (DMDS) on the cracking process, a solution to coke formation
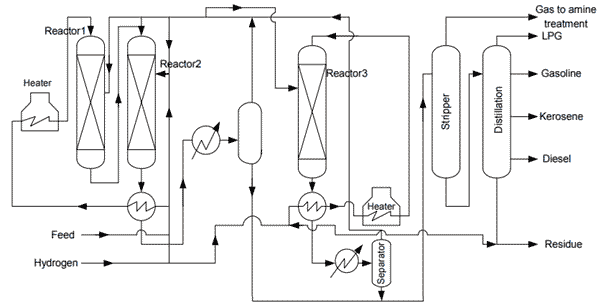
Catalytic cracking process was developed in 1920 by Eugene Houdry for up gradation of residue was commercialized latter in 1930. Houdry process was based on cyclic fixed bed configuration. There has been continuous up gradation in catalytic in catalytic cracking process from its incept of fixed bed technology to latter fluidized bed catalytic cracking (FCC).The feed stock for catalytic cracking is normally light gas oil from vacuum distillation column. Catalytic cracking, cracks low value high molecular weight hydrocarbons to more value added products (low molecular weight) like gasoline and LPG. The following figure show a simple schema from a cracking unit.
One of the compounds that is produced as a byproduct of some cracking reactions is hydrocarbons compacted polyaromatic, commonly known as coke. These deposits tend to lower the cracking activity of the catalyst and must be periodically removed by burning. Since coke represents a loss of desired products and subsequent regeneration supplies a major part of heat to the process, factors leading to coke formation are of great commercial importance. Coke formation occurs through three different mechanisms:
- Coke formation with catalytic mechanism
- Coke Formation with radical mechanism
- Coke formation due to condensation of polyaromatic compounds
Coke formation has a number of adverse effects in operational processes, including the following cases:
- Increased pressure drop on coils
- Hot spots phenomenon
- Coil corrosion phenomenon
- Reduce production
- Reduce selectivity
- Reduce rate of reaction
So, several methods have been presented to overcome the problems caused by the formation of coke during thermal cracking. These methods as follows:
- Upgrading of coils alloy to 35Cr / 45Ni
- Use of Anti Foulant Scale
- Adding sulfur compounds with decomposition properties at cracking temperature to feed
Adding sulfur compounds to feed is one of the conventional methods to overcome the effects of coke formation on cracking processes. The research results show among of 177 thermal cracking units in world, 144 cracking unit are using sulfur compounds to overcome the problem of coke production as well as increase the speed of cracking reactions. Among these sulfur compounds are sulfur, hydrogen sulfide, dimethyl sulfide, dimethyl disulfide, thiophene, dibenzyl sulfide, dibenzyl disulfide. Among Dimethyl disulfide (DMDS) is one of the most conventional sulfide compounds that used in olefins and other cracking units for this purpose. After addition DMDS, sulfur compounds lead to strong chemical absorption at the metal surface.
DMDS added in high temperature and then decomposed into carbonyl sulfide, hydrogen sulfide, dimethyl sulphide, methane, and SH radical, and thus the chemical absorption of sulfur at the metal surface creates a layer of metallic sulfide on the surface of the reactor and leads to the effect of this combination will be on the process. DMDS also used in the following reasons to reactors:
- Increased thermal cracking speed
- Increased selectivity of hydrocarbons conversion
- Prevent formation of coke at the internal surface of reactor coils
In Iranian refineries, DMDS is also used to prevent coke formation during the cracking process and increase rate of cracking reactions. Therefore, supplying DMDS is so necessary and crucial. Rayenehdaran Co. has been consistently trying to play an active role supplying DMDS with access to international recognized resources and manufactures.
References:
- Amano, T., Wilcox, J., Pouwels, C., “Process and catalysis factors to maximize propylene output”, Petroleum Technology Quarterly, 3, 2012, p.17.
- Debasis Bhattacharyya, “Fluid Catalytic Cracking: Process Fundamentals”, 6th Summer School on Petroleum Refining & Petrochemicals 6th–10th June, 2011.
- Ghosh, S “Fluid catalytic cracking: An overview & future scenario”, Indian Chemical Engineer special Issue, Vol 1,2002, p.27.
- Letzsch, W., “Improve catalytic cracking to produce clean fuels”, Hydrocarbon processing Feb, 2005,p.77.
- Jidong Wang, Marie-Françoise Reyniers, Bryan Marin, “Influence of Dimethyl Disulfide on Coke Formation during Steam Cracking of Hydrocarbons”, Article in Industrial & Engineering Chemistry Research, 46(12), January 2007.