The difference between ethanol and methanol
Ethanol or ethyl alcohol or ethyl alcohol or fruit alcohol has the chemical formula C2H5OH, which is a chemical compound with a specific odor and fire. Ethanol has been known to humans since ancient times, and its separation into relatively pure ethanol was probably first performed by Jabir ibn Hayyan, who expanded the distillation industry. Of course, it is more likely that pure ethanol was produced by the Iranian scientist Mohammad Zakaria Razi.
Methanol is the simplest alcohol, also known as methyl alcohol. Methyl alcohol has Greek roots and is made up of two words, Methu meaning wine and hyel meaning wood. Methanol is a chemical compound with the formula CH3OH and is a light, volatile and flammable liquid. Burning in the air, it produces carbon dioxide and water.
This compound is produced by the anaerobic metabolism of many species of bacteria, so there is a small amount of methanol vapor in the atmosphere, and the methanol in the atmosphere is oxidized to CO2 by oxygen and sunlight after a few days. Figure (1) shows the difference in molecular structure of ethanol and methanol.
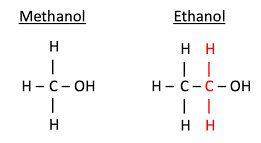
Fig (1): The difference in molecular structure of ethanol and methanol
Methanol has the weakest antimicrobial properties among alcohols; Therefore, it is rarely used for this purpose. In addition, methanol can be absorbed through the skin, respiratory tract, and gastrointestinal tract and converted into the human body as toxic metabolites of formaldehyde, formate (formic acid), and CO2. Not only is methanol highly toxic, it has the least antiseptic properties among alcohols, which can cause complications such as dermatitis (inflammation of the skin), eye damage such as blindness, kidney failure, coma and death.
The largest raw material for ethanol production in the United States is starchy vegetable products such as corn and sweet potatoes. After harvest, crops are sent to the plants in “wet mill” or “dry mill” plants. Both types of plants put the raw materials through the process of grinding, reaction and distillation, which at the end gives pure ethanol alcohol.
Pure methanol was first extracted from wood in 1661 by Robert Boyle. In 1834, the French chemists of the Jean-Babtist Society obtained the composition of its elements and also introduced the word methylene to organic chemistry. Methyl was derived from the word methylene in 1840 and was used to refer to methyl alcohol. In 1892, methyl alcohol was renamed methanol by the International Association for the Naming of Chemical Compounds.
In 1923, a German chemist named Matthias produced methanol from a synthetic gas (a mixture of CO and H2 obtained from coke). In this process, zinc chromate was used as a catalyst and the reaction was performed at very high temperature and pressure conditions.
The general process of methanol production can be summarized in the following three steps:
- Synthesis gas production
- Synthesis of methanol alcohol from the synthesis gas produced in the first stage
- Distillation
Although ethanol and methanol are both alcohols of the first group, they differ from each other in terms of production process, application and the degree of toxicity or not. The table below shows the differences between the two chemicals.
| Ethanol | Methanol |
| It has ethyl group. | It has a methyl group. |
| It is a weak acid compared to water. | Methanol has a higher acidity than water. |
| It has a pungent odor. | It is volatile and has a distinct odor. |
| It has a light blue flame. | When burning, the white flame lights up. |
| Produced by fermentation. | Produced through artificial processes. |
