Methylene Chloride
Methylene chloride was first formulated in 1839 by the French artistic chemist Victor Regna. To obtain methylene chloride, he first mixed chlorine and methyl chloride and then exposed it to sunlight. The main method of producing dichloromethane is that in the first stage, the reaction of hydrogen chloride and methanol leads to the production of methyl chloride. Excess methyl chloride mixes with the chlorine and reacts to produce methylene chloride, chlorophyll, and carbon tetrachloride as the product.
Methylene chloride is produced using chloromethane or by reacting methane with chlorine gas at a temperature of 400 to 500 ° C. At this temperature, methane and chloromethane undergo a series of reactions to produce products with a higher percentage of chlorine in their molecule. In 1993, 400,000 tons of methylene chloride were produced in the United States, Europe, and Japan. The reactions for the production of dichloromethane are given below:
CH4 + Cl2 -> CH3Cl + HCl
CH3Cl + Cl2 -> CH2Cl2 + HCl
CH2Cl2 + Cl2 -> CHCl3 + HCl
CH3Cl + Cl2 -> CCl4 + HCl
The output of these processes is a combination of chloromethane, dichloromethane (methylene chloride), chloroform and carbon tetrachloride. These compounds are separated by distillation. Methylene chloride mixture with air is stable and non-flammable. But the steam of this substance at high temperatures will be very dangerous. It is naturally present as a gas in seaweed, wetlands and volcanoes. Most of the gaseous methylene chloride in nature is due to human industrial activities.
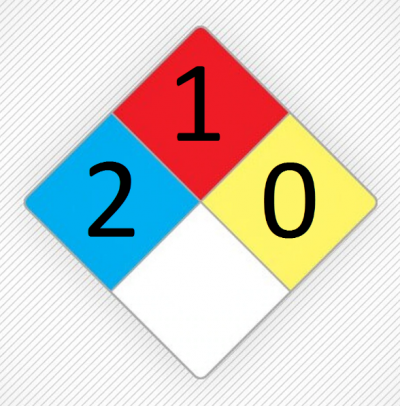
It is slightly toxic among chlorohydrocarbons. Nevertheless, it is still not safe. Methylene chloride has a high volatility that can be easily converted to steam. For this reason, the possibility of inhaling this substance is very high, which can cause harm to humans. It can also be absorbed through the skin. Excessive inhalation of this substance causes symptoms of decreased concentration, dizziness, nausea, weakness, numbness and dizziness. These are just some of the side effects of inhalation, and more serious side effects can include suffocation, coma, or even death. Figure (1) shows the NFPA for methylene chloride.
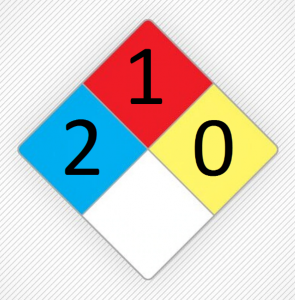
Figure (1): The NFPA for methylene chloride
Experiments show that this substance causes cancer of the lungs, liver and pancreas and is considered a carcinogen. It can also cause breast cancer and salivary gland cancer. Of course, it must be said that these experiments have been performed on animals and so far these cases have not been proven for humans. Inhalation of this substance is very dangerous for people with heart problems and can cause irregular heartbeats or heart attacks.