Isopropyl alcohol
Isopropyl alcohol is an organic compound also known as isopropanol and 2-propanol. This chemical compound is in the group of alcohol compounds and is highly flammable. Isopropyl alcohol was first produced in 1920 by Standard Oil by hydrating propene to oxidize acetone. Isopropyl alcohol liquid has a pungent odor, similar to all alcohols. The molecular formula of this organic compound is shown as C3H8O. Isopropyl alcohol is not oral.
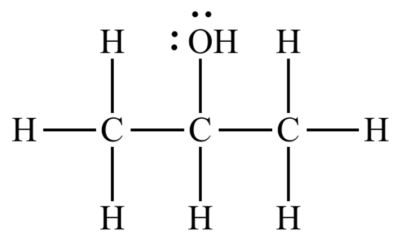
Isopropyl alcohol is metabolized differently in the human body than in other oral alcohols. As a result, drinking even a small amount of it can lead to irreparable and even fatal risks. In the molecular structure of this organic compound, the hydroxide group is located on carbon No. 2; thus, 2-propanol is a type 2 alcohol. Figure (1) shows the molecular structure of this alcohol.
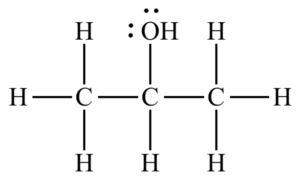
Fig (1): the molecular structure of Isopropyl alcohol
The United States, Germany, the Netherlands, Japan and South Africa are the largest producers of isopropyl alcohol in the world. Global demand for this substance is higher than other alcohols because isopropyl alcohol has a lower price after ethyl alcohol. Figure (2) shows the global isopropyl alcohol market based on the different classes of this chemical.

Fig (2): Global isopropyl alcohol market based on the different classes IPA
IPA production processes
There are two main methods for producing isopropyl alcohol, both of which use propene as a raw material. These two methods are:
- Direct flow of propylene with the help of homogeneous acid catalyst
- Indirect flow of propylene using a heterogeneous acid catalyst
IPA Applications
Most of the production volume of isopropyl alcohol is used for the production of agricultural chemicals, pharmaceuticals, process catalysts and solvents. Isopropanol is much easier to use in hydrogenation, oxidation, esterification, ether, amine, and halogenation reactions than the first type of alcohol, such as normal propyl alcohol or ethyl alcohol. Other uses for isopropyl alcohol include:
- Raw material of chemical industry: production of acetone and its derivatives, types of saline glass, disinfectants, dyes,
- Medical industry: Due to its antibacterial properties in soaps and lotions and in surgery as a skin disinfectant and the manufacture of antibiotics
- Fuel: As a fuel additive to prevent water accumulation in fuel pipes
- Laboratory Industries: As a disinfectant for cleaning equipment and surfaces
- Printing industry: as a solvent