Table of Contents
Injection of chemicals in membrane processes
In fact, a membrane acts like a barrier that separates the two phases and acts selectively against the transfer of different chemical species. The main factor of separation in membrane processes is the difference in the intensity of component transfer along the membrane. Membrane processes can be classified into three categories based on the driving force used in the process:
- Pressure driving force: Nano filtration, ultrafiltration, microfiltration and reverse osmosis
- Concentration difference driving force: dialysis
- Driving force of electric potential: electro dialysis
- Difference driving force: Membrane distillation
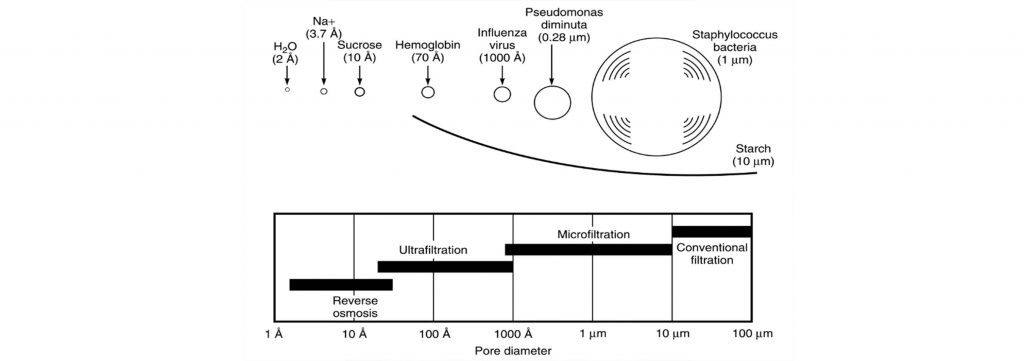
Porosity size range in three processes whose driving force is pressure (microfiltration, ultrafiltration and reverse osmosis) and their application in Water separation is illustrated in Figure (1).
Fig (1): Porosity size range in microfiltration, ultrafiltration and reverse osmosis
Membrane processes are divided into different types based on the driving force used in the system. An overview of the different membrane processes and driving forces is shown in Table (1).
Table (1): Classification of membrane processes based on driving force
| Driving Force | Product | Feed | Membrane Process |
| Pressure | Liquid | Liquid | Microfiltration (MF) |
| Pressure | Liquid | Liquid | Ultrafiltration (UF) |
| Pressure | Liquid | Liquid | Nano filtration (NF) |
| Pressure | Liquid | Liquid | Reverse osmosis |
| Pressure | Gas | Gas | Gas Separation (GS) |
| Pressure | Gas | Liquid | Membrane Evaporation (PV) |
| Concentration | Liquid | Liquid | Dialysis (D) |
| electrical charge | Liquid | Liquid | Electro dialysis (ED) |
Membrane Processes Advantages
Among the advantages of using membrane process over other separation processes are:
- Separation can be done continuously
- Energy consumption is generally low
- Membrane processes can be easily combined with other separation processes
- Separation can be done under mild conditions
- Easy scaling
- No additives required
- Membrane properties are variable and can be adjusted
Challenges facing membrane technology
One of the most important challenges in the field of separation using membrane technology is to increase the selectivity of a membrane so that its permeability is not reduced. Energy consumption is also important for the driving force in the process of water purification and desalination. Other challenges in the membrane separation process include the following:
- Resistance to fouling and clogged pores
- Mechanical stability
- Thermal stability
- Self-cleaning property
- Ability to simultaneously separate and destroy contaminants on the surface
- Quality and volume of effluent
Specifications and quality of reverse osmosis saline effluent flow
In reverse osmosis processes, the characteristics and quality of saline effluent depend on various factors. The most important of these factors are:
- Feed quality and specifications
- Quality of treated water flow
- Pre-treatment methods
- Chemicals and membrane cleaning methods
Feed quality of reverse osmosis system input
The characteristics of the input feed, including the following, play an essential role in the membrane separation process:
- Concentration (As the concentration increases, the amount of clogging in the process also intensifies.)
- Size and charge of particles in the feed (due to the load of the membrane causes it to be absorbed or repelled from the membrane surface)
- Viscosity (effective in fluid motion)
- Temperature (the most important factor affecting viscosity)
- Acidity (change in pH changes the surface charge of the particles)
The amount of electrical conductivity, total suspended solids and chloride ions in the effluent of reverse osmosis seawater desalination units is much higher than the effluent of industrial effluent treatment units. The amount of sulfate and chloride ions in the effluent of seawater desalination units is 2-8 times the effluent of industrial effluent treatment units.
While the amount of organic matter in the effluent of industrial wastewater treatment plants is higher. For this reason, reverse osmosis systems have a recycling rate of 40-40% for seawater treatment and between 95-65% for conventional saline water treatment.
Reverse osmosis system chemicals
The injection of chemicals in the reverse osmosis system is done in reverse osmosis unit in the following steps:
- Before refining the feed
- Membrane protection
- Chemical washing of membranes
- Final treatment of product flow and effluent
Table (2) shows the materials used in the reverse osmosis system.
Table (2): Common chemicals used in reverse osmosis units
| Typical Chemical | Pretreatment Process |
| Chlorine, sodium hypochlorite, calcium hypochlorite, ozone | Biofouling Control |
| Polymeric substances such as polyphosphates, phosphonates and polycarbonic acids | Antiscalant |
| Sulphuric acid, hydrochloric acid | Acid |
| Ferric chloride, ferric sulphate, polyelectrolytes | Coagulant /Flocculants |
| Sodium bisulphite | DE chlorination |
Pre-treatment
Ultrafiltration and microfiltration are among the most important membrane processes used to pretreat reverse osmosis membranes. At this stage, the particles that clog the reverse osmosis membranes are easily trapped and removed from the membrane chamber in reverse rinsing. The separation step using microfiltration and ultrafiltration membranes is similar to membrane processes such as reverse osmosis and nanofiltration, with the only difference being the size of the particles that are separated.
Ultrafiltration removes coarse molecules and particles with dimensions of 0.002, including colloids, proteins, microbial contaminants, and organic macromolecules, through which soluble materials and micromolecules pass. While microfiltration removes suspended particles and large colloidal particles with dimensions of 0.1 to one micron.
The solid particles present in the washing flow of these filters, due to the addition of coagulants before these filters, are 60 to 80% higher than the washing flow of the filters. In fact, fluid pretreatment is to change the properties of the fluid so that clogging is minimized. The main purpose of the pre-treatment system includes the following:
- Prevent oxidation and hydrolysis of membranes
- Separation of metals and chemicals from feed
- Reduction of filling due to suspended particles
- Microbial growth of inorganic sediments due to insoluble salts and silica
Some common pre-treatment methods are:
- Use of filtration (usually coarse particle separation)
- Chemical treatment (coagulation, flocculation or removal of solid particles)
- Adjust pH
Biological clogging control
Biological control is to prevent the formation of microorganisms and the emergence of biological clogging. Chlorine in the form of gaseous chlorine, sodium hypochlorite and ozone are used as oxidizing agents throughout the pretreatment step. At the end of the pretreatment process, due to the sensitivity of polymer membranes to chlorine, this material must be separated.
Ultraviolet light can be used as an alternative to chlorinated materials, which has less effect on the chlorination process than chlorinated materials and ozone, and does not require final separation with reducing agents.
Acid injection
In order to increase the solubility of calcium carbonate, reduce the amount of sediment on the surface of membranes and membrane clogging, the pH of the input feed is lowered by adding suitable acids. Acids improve colloid coagulation and slightly increase the solubility of silica. Among the most common acids used in the pretreatment process are sulfuric acid and hydrochloric acid.
Injection of coagulants and flocculants
Coagulants are used to bond colloidal particles together to form larger particles. In the pre-membrane filtration stage, they must be separated from the feed. Therefore, in reverse osmosis system, effective method of separating coagulants is necessary to prevent clogging of membranes. These materials can be used organically or inorganically and enter the effluent stream of the reverse osmosis system along with the washing of the filters.
Injection of anti-fouling material
Clogging of membranes due to the deposition of insoluble salts in the feedstock limits the percentage of recycling in reverse osmosis systems. Injection of sediment inhibitors is done to minimize the possibility of sediment formation.
Chemicals for washing membranes to prevent clogging
Clogging is the accumulation of material on the surface or in the pores or cavities of a membrane so that its mode of action changes. Accumulation of materials and their adhesion causes the formation of layers on the surface of the membrane, which increases the resistance to fluid passage. In this way, the outflow flux from the membrane is gradually reduced and the problems caused by clogging are:
- Reduce flux
- Decreased solute excretion
- Reduce system efficiency
- Increase the pressure required to perform the process
There are four main categories for Membrane clogging:
- Colloidal clogging (including accumulation and adhesion of colloidal particles and materials)
- Biological clogging (adhesion and growth of biofilm)
- Organic clogging (adsorption of certain organic compounds such as humic substances and oils on the membrane surface)
- Inorganic clogging or fouling (precipitation of soluble salts)
Table (3) shows the materials used to prevent membrane clogging.
Table (3): Used to prevent membrane clogging
| Typical cleaning chemicals | Foulant |
| Citric acid, phosphoric acid | Metal oxides |
| Sodium hydroxide, sodium dodecyl | Inorganic colloids |
| Sodium ethylenediamine tetra acetic acid (Na-EDTA), sodium triphosphate, trisodium phosphate | Biofilms
|
| Sodium hydroxide, sodium dodecyl sulphate, sodium dodecyl benzene sulphonate | Organic matter
|
| Ammonium bifluoride, sodium hydroxide, sodium dodecyl benzene sulphonate | Silica
|
Sedimentation also includes calcium carbonate, barium sulfate, calcium sulfate, strontium sulfate and calcium fluoride. Commonly used materials for chemical washing include:
- Acids
- Bases
- enzymes
- Surface activating agents
- Antiseptics
- Some simple compounds such as water
In general, acidic substances are used for mineral-induced clogging and alkaline materials are used for organic and biological clogging. Due to the alkaline nature and acidity of the materials used to wash the membranes, they must first be neutralized and gradually added to the effluent flow of the reverse osmosis system.
Although the use of the above cases significantly reduces membrane clogging, but does not completely prevent membrane clogging, and eventually membrane clogging occurs.
Inlet streams to the reverse osmosis unit effluent
Two streams are added to the reverse osmosis system effluent stream, these are:
- The initial flow of the reverse osmosis system, which does not yet have the desired quality to enter the membranes. This flow is similar to the input feed stream in terms of salt concentration.
- Refined osmosis system refined product flow that does not yet have the proper quality of the final product of the reverse osmosis system. The concentration of salts in this stream is less than the flow of incoming feed.