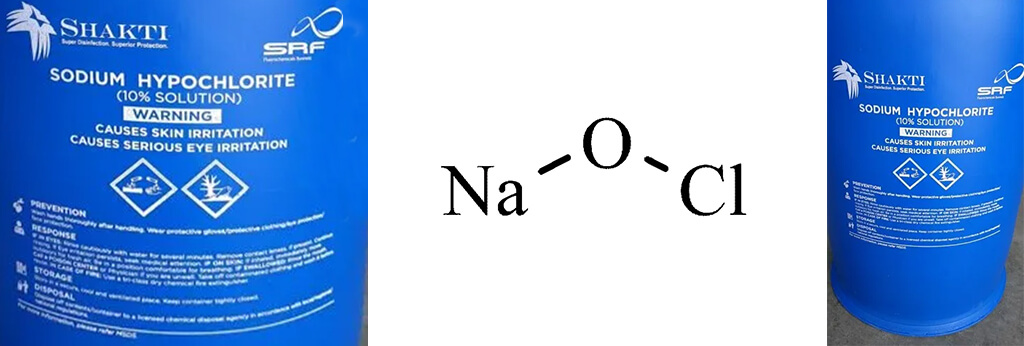
Sodium hypochlorite is a chemical substance with the formula NaClO. The solution form of this substance is available as a bleach under the brand name of Bleach. Bleach, which is also called bleaching liquid and Vitex that is used for disinfection and deodorization.
By dissolving salt in water, the solution is electrolyzed and forms sodium hypochlorite solution in water. This solution contains 150 grams of active chlorine (Cl2) per liter. During this reaction, explosive hydrogen gas is also formed. Also, by adding chlorine gas (Cl2) to caustic soda (NaOH), sodium hypochlorite, water and salt (NaCl) are produced.
Characteristics
| Molecular mass | 74.442 g/mol |
| Melting point | 18 °C |
| Boiling point | 101 °C |
| Appearance | greenish-yellow solid |
Packing
Sodium hypochlorite is supplied in bulk in polyethylene tanks or in 220 liter HDPE barrels.
Application
- Textile and paint industries
- Detergent industry
- Petrochemical industry
- Water and wastewater treatment
- food industry
- agriculture
- Glass industry
- Paper industry
Other Names
- Javel water
- bleach
- chloride of soda
- antiformin
Chemical Formula
NaClO
