Boric acid
Boric acid is a chemical with the formula H3BO3 or B (OH)3 which is found as a crystalline solid. Boric acid is also known by other names such as hydrogen borate, orthoboracic acid, boracic acid, orthoboric acid and Lewis boric acid. Boric acid crystals are colorless and white. This chemical is odorless and has a bitter taste. Boric acid is soluble in water and dissolves well in boiling water. This mineral is melted at a temperature of 236 ℃ and hydrated at a temperature of 300 ℃ and forms tetra boric acid or pyroboric acid. This chemical is very resistant to flame and is not flammable. Figure (1) shows the molecular structure of boric acid.
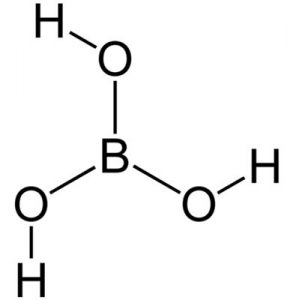
Fig (1): Molecular structure of boric acid
Boric acid is a weak acid and its purity cannot be determined by simple titration using sodium hydroxide. The purity of this acid can be determined by titration with a standard solution of sodium hydroxide in the presence of polyhydric alcohols such as glycerol. Boric acid glycerol acts as a strong acid and its titration with sodium hydroxide in the presence of a phenolphthalein detector is effective in determining the purity percentage. The purity of this product from boric acid is 90%.
Boric acid production process
This compound is present in nature in the form of sassolite mineral and is found near volcanoes and hot springs as a white or yellow solid.
Boric acid is present in free form in some volcanic areas such as Tuscany in Italy and Lipari in Iceland, where it is found along with steam in cracks in the earth.The main composition of colemanite mineral is boron oxide, which is a mineral compound
It is also found as a constituent of many naturally occurring minerals – borax, boracite, ulexite (boronatrocalcite) and colemanite. For this purpose, the desired mineral is reacted with sulfuric acid to separate the boron-containing compounds from the minerals. The boron solution is separated from the other suspended solids in the suspension and then hydrogen sulfide is added to the solution to precipitate the arsenic and iron impurities in the solution.
Boric acid is a weakly acidic hydrate of boric oxide, first developed by Wilhelm Homberg from the reaction of borax with mineral acids. The acid is often used as a disinfectant, insecticide, in agriculture, in the production of fire suppressants, and as a raw material in the production of many chemical compounds.
There are two general methods for synthesizing boric acid:
First process:
This process is actually the reaction that Wilhelm used to produce orthoboric acid. This method uses the reaction of borax with mineral acids such as hydrochloric acid to produce boric acid under certain conditions. In the laboratory, 12 grams of borax is first dissolved in 25 ml of warm distilled water. Neutralize the precipitated solution with 25% hydrochloric acid and allow the solution to cool slowly. After crystal formation, the crystal is smoothed with a Buchner funnel. Finally, the boric acid produced is poured on filter paper and allowed to dry. The percentage of boron in boric acid is about 18%.
Na2B4O7·10H2O + 2 HCl → 4 H3BO3 + 2 NaCl + 5 H2O
Second process:
In the second method, this compound is prepared as a by-product of the hydrolysis of boron trihalides and deborans.
B2H6 + 6 H2O → 2 H3BO3 + 6 H2
BX3 + 3 H2O → H3BO3 + 3 HX (X = Cl, Br, I)
Global market for boric acid
China and the United States are among the countries with the highest levels of boric acid consumption. There are many sources of borax ore in Turkey and the United States, and large producers of acid are located in these two countries. In China, the use of this product has increased due to the growth of the textile industry, electronic printed circuit boards and shipbuilding. Forecasts in the global boric acid market show that the market for this substance will grow during the years 2020-2030. Figure (2) shows the growth trend of boric acid market by 2030.
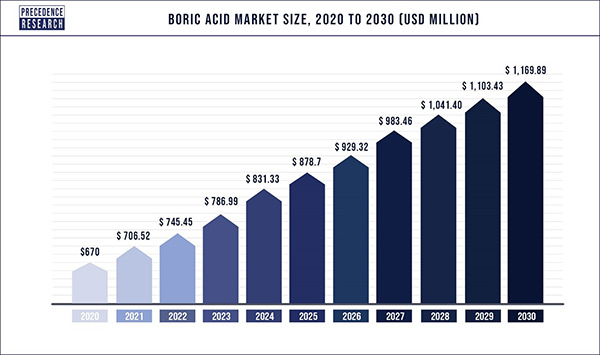
Fig (2): Predicting the upward trend of boric acid market by 2030
Safety
Gloves, goggles and masks should be used when using boric acid, and in case of contact with the body, it should be washed with plenty of water and a doctor should be consulted if necessary. Boric acid should never be used orally, on open wounds and in children.
In order to use it for rinsing the eyes, you should act with caution and be sure to consult your doctor about this.
Symptoms of boric acid poisoning include blue or green vomiting, coma, drowsiness, and low blood pressure.
And in some cases, muscle contraction, also if boric acid consumption causes a large number of holes in the esophagus or stomach may cause death. Figure (3) shows the NFPA boric acid rhombus.

Fig (3): NFPA boric acid rhombus
Boric acid usage
The compound has been widely used since the Greeks and Romans used it for disinfection and cleaning. Based on the market area, this compound is divided into Asia-Pacific, North America, Europe, South America and the United Arab Emirates, in each of which the major applications in agriculture, medicine and pharmacy, and nuclear research determine its market. are. Figures (4) and (5) show how boric acid is distributed in different parts of the world and how boric acid is distributed in different industries, respectively.
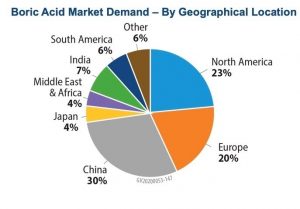
Fig (4): How to distribute boric acid consumption in different regions of the world
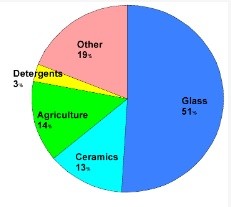
Fig (5): How to distribute boric acid consumption in different industries
important uses
The following are the most important uses of this chemical:
Production of insecticides
Boric acid was first used in insecticides to repel beetles, termites, fleas and others. As a stomach toxin, it affects insect metabolism, and its dry powder is corrosive to the exoskeleton of insects. The above product and its sodium salts such as borax decahydrate, borax pentahydrate, borax anhydrase, etc. can be used in the structure of pesticides.
Tile industry
The main application and application of boric acid is in the ceramic tile industry and the production of refractory materials
Medical
As a disinfectant for burns or minor cuts, treatment of fungal infections is also very effective in burns ointments or treatment of minor cuts.
Wood protection
The combination of boric acid with the ethylene glycol carrier is used to prevent fungal and insect infestations in order to prevent damage to the wood.
PH adjustment
Boric acid is in equilibrium with its conjugate base, or borate ion, and is widely used as a buffering system. One of the uses of boric acid as a buffering agent and pH regulator is in controlling the acidity of pool water.
Agriculture
Boric acid fertilizer is used to prevent boron deficiency in plants. It is also used to preserve grains such as rice and wheat. This compound is an excellent disinfectant to kill bacteria and fungi. Boric acid provides the boron element needed by pistachio trees. Adequate boron in the soil for pistachio trees causes better growth, fewer pests and better quality crop. However, excessive use of this acid causes soil poisoning and reduced plant growth.
Nuclear power plant
The substance is used as a neutron toxin in some nuclear power plants. The boron in this compound reduces the chance of thermal fission by absorbing some thermal neutrons.
Lubrication
Colloidal suspensions of oil-soluble orthoboric acid nanoparticles can produce significant lubricating properties on ceramic or metal surfaces.
Chemicals
Boron is used in the chemical industry to prevent the amide formation reaction between aluminum and nitrate.
Making fiberglass fabric
Boric acid is a single-strand fiberglass fabric used to reinforce plastics used in industrial plumbing, boat building, and computer circuit boards.
Leather industry
In tanning, they improve damaged cow skin by adding boric acid to the salt used for salting and disinfection. Also, since this acid is able to control and reduce the growth of bacteria, its use in the leather industry also helps to repel insects and a variety of microorganisms.
Gold industries
In the jewelry industry, this acid is used in combination with denatured alcohol or ethanol to reduce oxidation of metal surfaces and prevent the formation of oxide layers on them.
Making LCD
This weak acid is also used to make glass for flat and LCD displays.
supplements
Boric and other borates are increasingly used as a source of boron in supplements without supplements. Boron is thought to have therapeutic value in promoting bone and joint health and also has a limiting effect on the symptoms of osteoarthritis. It is important to note that the health effects of boron supplements are based on very recent studies or on the claims of supplement manufacturers.
Prevent flame propagation
Boric acid prevents the release of combustible gases from some materials such as cotton, wood and paper products. The chemical bond also releases water to prevent combustion.
Glass industry
Boric acid and other borates in the glass industry increase the chemical resistance and stress resistance of glass. The resistance of halogen lamps, cookware, microwave ovens, laboratory utensils and many glassware increases with the addition of boric acid.

