Benzoyl Peroxide (1)
Benzoyl peroxide is a chemical compound with the chemical structure (C6H5CO)2O2. This substance is also known by the acronym BzO2 or BPO. It is a white granular crystalline solid with a faint odor of benzaldehyde that has little solubility in water but is soluble in other solvents such as acetone, ethanol and many other organic solvents. Another name for benzoyl peroxide is di-benzoyl peroxide, and benzoyl peroxide is a component of acyl peroxides in the category of organic peroxides. Acyl peroxides are a good source for the production of free radicals in chain polymerization reactions. Figure (1) shows the molecular structure of dibenzoyl peroxide.
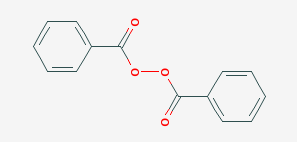
Fig (1): Molecular structure of dibenzoyl peroxide
Benzoyl peroxide is a chemical that is used in industry in four ways, which are:
- Benzoyl peroxide aqueous powder
- Benzoyl peroxide powder without water
- Suspended benzoyl peroxide paste
- Liquid benzoyl peroxide dissolved in organic solvents
According to Exonobel’s proposal for benzoyl peroxide powder, the best storage temperature is below 25 degrees Celsius so that benzoyl peroxide powder can maintain its quality well. As mention at before the chemical structure of benzoyl peroxide is , to which two benzoyl groups are attached to the peroxide group. The spontaneous decomposition temperature of benzoyl peroxide powder is about 55°C and should be kept below this temperature to prevent explosion and fire hazards. From the decomposition of benzoyl peroxide, the main chemical compounds that are produced are:
- Carbon dioxide
- Benzene
- Benzoic acid
- The phenyl
- Phenyl benzoate
Benzoic peroxide production process
One of the following two processes is commonly used to produce benzoic peroxide:
First process:
Production of benzoyl peroxide from benzoyl chloride and barium peroxide using the following reaction:
2C6H5COCl+BaO2 -> (C6H5CO)2O2+BaCl2
Second process:
Production of benzoyl peroxide Reaction between benzoyl chloride with oxygenated water (hydrogen peroxide) and sodium hydroxide using the following reaction:
2C6H5COCl+H2O2+2NaOH -> (C6H5CO)2O2+2NaCl+2H2O2
