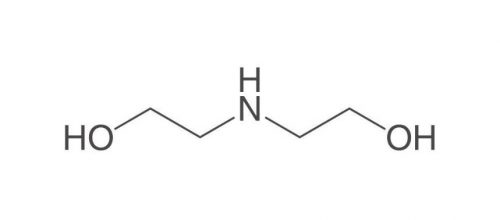
Diethanolamine (DEA)
Diethanolamine, abbreviated DEA, is a colorless, ammonia-like liquid with the chemical formula C4H11NO2, an organic compound consisting of a second-class amine and a dual alcohol. This substance is weakly related to other amino acids such as monoethanolamine or triethanolamine. This dual alcohol has two hydroxyl groups and, like other amines, diethanolamine acts as a weak base. This product decomposes due to heat and produces toxic fumes. It is soluble in water and by dissolving this substance in water, a solution with almost strong base power can be obtained. Diethanolamine reacts with metals such as copper, zinc, aluminum and their alloys and causes corrosion of these metals. Reacts strongly with strong oxidants and strong acids. Figure (1) shows the molecular structure of diethanolamine.
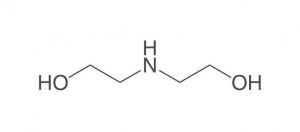
Figure (1): Molecular structure of diethanolamine (DEA)
Global market in ethanolamine
Between 2012 and 2017, the production capacity of ethanolamines around the world increased significantly, in which China played an important role, and continued to expand its capacity to reduce its dependence on imports and meet the growing demand for this The batch of ingredients continues. Consumer markets with the highest growth potential for these products are predicted to include herbicides (for DEA), ethylamines and triazines (for MEA) and esters (for TEA).
Diethanolamine production process
To produce ethanolamines, ammonia solution, ethanolamines, and ethylene oxide are continuously and intermittently introduced into the reaction system. The following reaction indicates the desired reaction:
C2H4O + NH3 -> H2NCH2CH2OH
First, monoethanolamine is produced by the reaction of ethylene oxide with ammonia in the aqueous phase, after which the reactions continue as follows to produce diethanolamine and triethanolamine:
C2H4O + H2NCH2CH2OH -> HN(CH2CH2OH)2
C2H4O + HN(CH2CH2OH)2 -> H (CH2CH2OH)3
Application of diethanolamine
One of the most important applications of diethanolamine and amine in general is the use in gas sweetening process. Removal of acidic gases such as CO2, H2S, COS and CS2 from natural gas is one of the basic and important operations in industrial processes. These acidic gases can all be present in natural gas, but the most common is hydrogen sulfide. Amine compounds are used to remove these impurities during the sweetening process.
- Cosmetics: Production of shampoos and cosmetics as a foaming agent
- Agricultural pesticides: as a neutralizer of agricultural chemicals
- Production of surfactants: surfactants in detergents and hygiene
- Production of industrial fluids: in combination with acids such as oleic acid or carboxylic acid
- Textile industry: As a softener in the textile industry
- Catalyst: As a catalyst in the production of flexible and inflexible polyurethane foams
- Rubberization: The raw material for the production of alkanoamide as a curing agent and the distribution of fillers in rubber