Production of base oils using solvent extraction method (part 1)
In refining the base oil using acid, chemical reactions are used to reduce the aromatic components and eliminate the reactive components, reducing the useful life of the oil, but in the extraction method, physico-chemical separation is performed using a solvent. In this way, with the help of extraction method, most of the aromatic compounds in the oil are extracted using a solvent, and after the solvent is recycled, it is extracted from the unit.
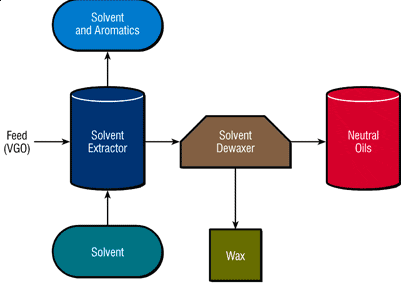
The product of this stage, which is sent to the next stages for further refining, is called refinite. Rafinite has a higher resistance to heat and oxidation and its viscosity index is higher than the input feed to the solvent extraction unit. In particular, the selectivity for multi-ring aromatic compounds with three or more rings has special attention due to the carcinogenicity of these compounds. Figure (1) shows the oil production process using the solvent extraction method.

Figure (1): Extraction of base oil using solvent
In the extraction process using a solvent, the solvent and the feed inlet are mixed in the column and the mixture remains almost constant for a short time. As a result, aromatic substances dissolve in the solvent and form two phases. The phase with aromatic substances has a dark color and the other phase has a light color.
In general, the solvent extraction process is only able to produce low quality final base oils and the properties of the produced oil are highly dependent on the type of feed of this unit. The choice of solvent used in this method depends on the following factors:
- Selectivity (leads to the production of high quality raffinite)
- Solvent absorption (to minimize solvent to oil ratio)
- Ease of solvent recycling (solvent boiling point should be less than the boiling point of raffinate and separated aromatic substances)
- Suitable properties of the solvent (these properties include: high stability, safety, low toxicity, easy attack and reasonable price)