Potassium Chloride (KCl)
Potassium was discovered in 1807 by davy humphry which obtained it from caustic potash. This alkali metal was the only metal that was separated by electrolysis. Potassium chloride is a type of salt that is formed from a combination of the metal potassium and chlorine halogen. Potassium chloride is a type of salt that is formed from a combination of the metal potassium and chlorine halogen.
Among the common chemicals in the oil well drilling industry is potassium chloride solution, which is used for reasons such as low cost and easy access, compatibility with drilling fluid additives, stabilization of loose and water-sensitive clay, and rapid dissolution. is Common. Figure (1) shows how potassium chloride chemical is distributed in the world in 2017.

Fig (1): Distribution of potassium chloride in the world in 2017
In the process of producing potassium chloride, the ore is concentrated and processed to remove impurities and to obtain potassium chloride as the final product. In order to produce potassium chloride, two types of dry and wet processes are usually used. In the process of dry production of potassium chloride, the following steps are performed:
- Crushing
- Separation of slurry containing mineral
- Dual salt separation
- Separation of potassium chloride from a combination of two salts
- Drying
- Sorting
The potassium chloride produced by the process is supplied in three different forms:
Granules: About 70% of the total potassium chloride produced is consumed as a mixture of dry matter.
Standard: This type is used as a suspension in chemical fertilizers.
Soluble: Used in soluble fertilizers.
Figure (2) shows the distribution of potassium chloride consumption in different industries. As can be seen in this figure, the highest consumption of potassium chloride is related to the use of this substance in the production of co.
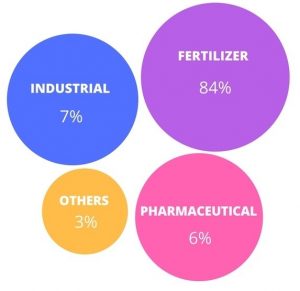
Fig (2): Distribution of potassium chloride consumption in different industries
Most important applications of potassium chloride include the following items:
Agricultural Industries: Potassium fertilizers are the third most consumed in terms of consumption after nitrogen and phosphorus
Alloy: Sodium and potassium alloy due to its high heat transfer characteristics
Food industry: Potassium nitrate as a food preservative
Pharmaceutical industry: Production of potassium salts orally or by injection