Oxidation Process (1)
The oxidation process was introduced in 1987 by Glaze et al. By definition, advanced oxidation refers to the processes in which free radicals, especially OH hydroxyl radicals, are sufficiently generated to oxidize non-degradable organic chemicals in the reaction medium under environmental conditions. Hydroxyl radical is a highly active oxidant that attacks most organic matter.
The reaction kinetics is usually primarily relative to the concentration of the hydroxyl radical and to the concentration of the oxidizing substance. The mechanism of oxidation of substances with OH depends on the type of organic matter.
The oxidation process is based on how the OH radical is produced in the oxidation reaction. Treatment of effluents containing various contaminants such as the following compounds has been investigated by photocatalytic oxidation method in various studies:
- Phenolic compounds
- Alcohols
- Organic acids
- Sulfur hydrocarbon compounds
- Pesticides and insecticides

Processes that use the effect of ultraviolet light on the performance of catalysts under reaction conditions are referred to as photocatalytic reactions. One of the main characteristics of light-sensitive catalysts is their semiconductor properties. Understanding photocatalysts requires familiarity with the nature of semiconductors.
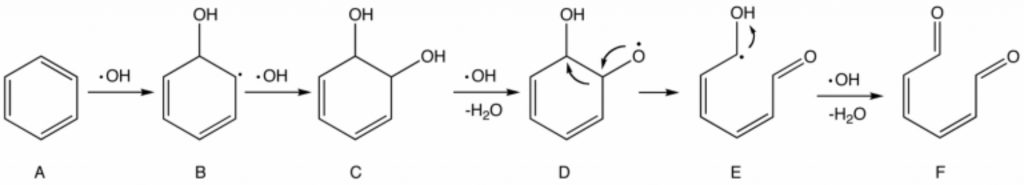
In the early stages of advanced oxidation processes, it leads to the production of hydroxyl free radicals, which with the high oxidation potential have the highest efficiency among other oxidants in the oxidation of organic compounds.
The lifespan of a hydroxyl radical is very short, but it is so strong in terms of oxidizing potential that it can easily oxidize an organic compound by absorbing hydrogen. The mechanism of hydroxyl radical reaction with organic compounds is very complex. This mechanism and radical reactions in general can be summarized in three steps:
- The initial or initial reactions in which free radicals are produced at this stage.
- The diffusion reactions in which the radicals produced from the previous stage are converted to other free radicals.
- Final reactions in which the free radicals of the previous stage become a stable compound.
From a kinetic point of view, reactions related to OH radicals are much faster than other oxidants. However, due to the radical reaction of hydroxyl with organic compounds, it produces a large number of intermediate compounds, which themselves react with the OH radical as a competitor of the primary compounds and act as a so-called OH radical scavenger. Therefore, complete degradation of mineralization – an organic compound – will be achieved under special conditions where the OH radical concentration is very high and the reaction time is high enough.