Olefins are not naturally present in crude oil but are produced during refining processes. Olefins are structurally very similar to paraffins, but in their structure, at least one carbon-carbon double bond is seen, and their general formula is CnH2n. Typically, Olefins are not ideal products in end products because they are highly reactive, double-bonded, and easily oxidized and polymerized.
Olefins include a variety of products due to the reactivity of their compounds. Light Olefins account for more than 60% of the petrochemical base material production in the world, and due to the very diverse use of Olefin products in daily life, the demand for this category of products in the world is constantly increasing.
All petrochemical products are produced from the basic materials like Ethylene, Propylene, Gases, Naphtha and so on. Among the main petrochemical base products, three products, namely propylene, ethylene and butylene, are Olefin products, which together account for 57% of the base production products in the world.
Water pipes, all kinds of polymers for medical purposes, all kinds of packaging products, parts needed in the automotive industry, and many things called plastics in everyday life are based on ethylene and propylene, all of which are Olefins.
Olefin production processes
Olefins are considered to be key components of the chemical industry. Ethylene and propylene are the most important Olefins, with an annual production of roughly 1.5×108 t and 8×107 t, respectively. These production rates are expected to increase as a result of an increasing global population combined with rising living standards. Light Olefins are the mainstay of modern life, as many different derivatives used in our daily lives are produced from these building blocks.
Traditionally, Olefin production depends mainly on natural gas processing products or crude oil fractions. The current leading technology for Olefin production is steam cracking (SC). In this process, hydrocarbons that primarily originate from fossil resources are cracked at elevated temperatures in tubular reactors suspended in
a gas-fired furnace.
In recent decades, this process has been highly optimized and its capacities have been increased, resulting in a well-established technology whose economics can hardly be challenged. Declining crude oil reserves and increasing social awareness of the human impact on the environment have had very little impact on the petrochemical industry.
Investments in alternative processes and feedstocks are still to come; the lack of economic viability of such processes in an uncertain commodity market threatens their large-scale implementation. However, some exceptional cases have showed economic viability as a result of limited supply, or have benefited from favorable policies.
A continuing search for alternative and preferably also more sustainable processes and feedstocks will eventually be required in order to fulfill the future demand for commodity chemicals. Potential alternative feedstocks are coal, natural gas, biomass, waste streams, and their derivatives.
Thanks to technological advancements and refinements in cracking, the methane supply has increased enormously since 2008 and the price of methane has dropped significantly. This makes shale gas a game changer and an interesting cost-competitive feedstock.
The large availability of shale gas has reinforced the interest in routes for valorizing methane in the form of Olefins and higher hydrocarbons, either directly or indirectly. These methane-conversion processes could be an excellent way to valorize large amounts of methane from stranded gas rather than from shale gas.
Although the growth of shale gas has triggered increasing research into methane upgrading processes, methane from shale gas is relatively expensive due to stringent specifications on maximum ethane content. This has caused the price of ethane to drop significantly, falling even below its calorific value. The abundance of cheap ethane created by shale gas exploitation has enabled cheap low-Olefin production via SC and has had a profound impact on the local US Olefin market.
Many newly built crackers are ethane based, and many existing liquid crackers are retrofitted to lighter gaseous feeds, as such feeds offer an economic advantage when accessible. New ethane steam crackers are expected to come online soon and to add 1×107 t of ethylene capacity by 2020 in the United States.
Globally, ethylene producers will experience a substantial capacity expansion in the next five years, with an annual increase from 1.76×108 t to 2.18×108 t and with investments of nearly $45 billion USD in upcoming projects. These large investments suggest that SC of hydrocarbons will remain the main pathway in the production of light Olefins. Ethane cracking is highly selective toward ethylene; hence, few or no other Olefins are coproduced. Therefore, the shift toward lighter feedstocks has resulted in increasing interest in developing on-purpose production routes toward propylene and higher Olefins.
On the other hand, direct SC of crude oil is also gaining importance. Using this process, petrochemical producers can skip the refining step and thus reduce their production cost. However, the need for cleaner air and water and for environmental protection will eventually lead to the exploration of sustainable production possibilities, accelerated and activated by political decisions.
Researchers and engineers are expending considerable efforts to explore and optimize these alternative production possibilities, in an attempt to increase both efficiency and profitability. Nevertheless, still more effort is absolutely necessary in order to take full advantage of sustainable or alternative feedstocks and technologies.
Reliable fundamental multi-scale models need to be developed and enhanced in order to explore unprecedented levels of efficiency, increase process maturity, and address major bottlenecks.
In this respect, fast implementation at a lower risk can be made possible. Aside from obtaining a fundamental understanding of the new processes, the end-goal of these efforts is to face the fact that environmental resources are limited and to secure future needs in terms of energy and chemicals.
However, the use of fossil feedstocks as a fuel or for the production of chemicals will remain dominant in the near future. This contribution focuses on promising Olefin production technologies that are most likely to challenge the current leading technology. Each of these alternative Olefin production pathways benefits from the abundance of propane, ethane, and methane that is available from shale gas and stranded gas.
Furthermore, the relevance of each pathway could be enlarged, as the shift toward lighter feedstock utilization in the SC of hydrocarbons results in the decreased production of important coproducts. Pathways that adopt renewables or waste streams will not be addressed in this perspective because they are believed to have rather low significance for the total quantity of Olefins produced in the near future.
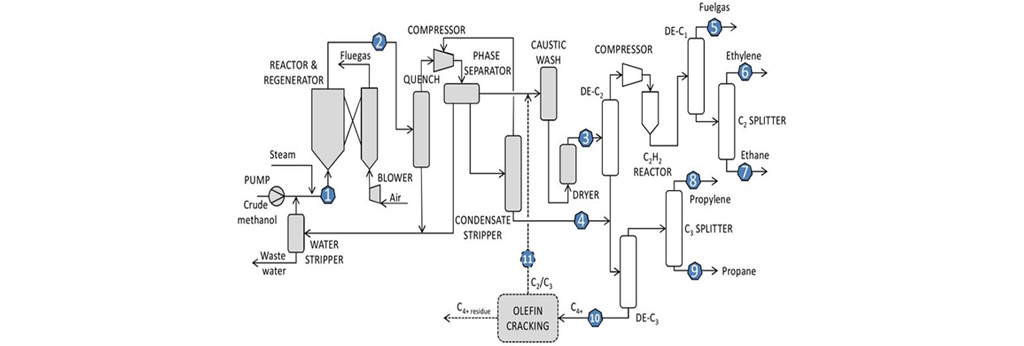
The following technologies are of interest: the catalytic dehydrogenation of light alkanes, the oxidative coupling of methane (OCM), and syngas-based routes such as the Fischer-Tropsch synthesis (FTS) and methanol synthesis followed by methanol to Olefins (MTO). Figure 1 shows a simple diagram of the Olefins production process.

Simple diagram of the Olefin production
Olefin process feed
There are three ways to produce light Olefins around the world:
• Coal
• natural gas
• Crude oil
Coal
The Chinese are the largest consumers of petrochemical products in the world, producing methanol due to lack of access to natural gas resources from coal, and then using processes to convert them into Olefin products. Of course, in recent years, the Chinese have always invested in natural gas extraction projects and Olefin petrochemical units in other countries, and given the wide range of uses of petrochemical products, the supply of these materials is necessary and strategic for them.
Natural gas
Natural gas is the best and cleanest way to get Olefin products that are cheaper and more economical than others. Among the countries with natural gas, Qatar has invested heavily in petrochemical products and it can be said that our country still has a lot of space to invest in this sector and Olefin products can transform the export of Iranian petrochemical products.
Crude oil
Another method is to produce Olefins from crude oil, which many oil-rich countries, especially Saudi Arabia, have focused on, producing large quantities of ethylene. Among Olefinic materials, ethylene is very popular and accounts for more than 50% of Olefin base products.
One of the most important concerns of the petrochemical industry in the world is optimization and selection of appropriate and low-cost methods for Olefin production. Iran, with all the necessary reserves for the production of Olefin products, is one of the pristine and suitable countries for investment in this sector.
Among the world’s latest technologies for the production of Olefin-based products is the conversion of propane to propylene and methanol to Olefins, which shows the special place of catalysts in this industry. Figure (2) shows the production of light Olefins in the world.
Light Olefins
Light olefins containing the following compounds:
• Two carbon – ethylene
• Three carbon – propylene
• Four carbon – butadiene and butylene
The above compounds are unsaturated due to having at least one carbon-carbon double bond and are considered reactive compounds.
The constituent parts of the Olefin unit
The following sections relate to an Olefin process in an ethane cracking unit:
• Cracking furnaces
• Hot section
• Density section
• Ethylene recycling and purification
• Cooling systems
• Water vapor, Blow Down, cooling water, gas fuel and other ancillary services
• Product storage in tanks
• Storage chemicals for your crushing unit
• Feller
• Cracking furnaces
The thermal refractory furnace is the heart of an Olefin unit. In fact, the heat required to carry out the reactions is provided by the thermal break furnace in which the reactor is located.
The main feature of hydrocracking reactions is the breaking of the carbon-carbon bond. Depending on the status of this link, the reactions can be divided into three groups:
• Simple hydrocracking reaction (C-C failure in a chain):
This reaction is the sum of two reactions of chain hydrocarbon cracking and hydrogen-derived Olefin saturation:
![]()
• Dealkylation reactions in the presence of hydrogen (C-C failure adjacent to a ring):
![]()
• Loop opening reaction (C-C failure in a loop)
![]()
The basic reaction governing the cracking of heavy sections consists of saturated aliphatic organic hydrocarbons in the form of paraffin and Olefins (Figure 3 Reaction I). This cracking is called “primary” cracking.

Major reactions in pyrolysis of hydrocarbons
Secondary cracking reactions (reactions I and III) produce a series of light products, other than Olefins, the composition and amount of which depend on the operating conditions.
Complete dehydrogenation reactions of Olefins lead to the formation of highly unsaturated compounds such as acetylene, which are present as undesirable impurities in the Olefinic compounds C2 and C3, or dolphin derivatives (IV reaction) that exert their activity. In fact, the latter reaction is the reverse of the cracking mode and produces heavy products by the Diels & Alder reaction or cycling.
Compounds that are formed in this way and dehydrogenation continues at the same intensity (reaction IV) leads to the production of a series of aromatic hydrocarbons, especially benzene, which are dense polyaromatic hydrocarbons, which are called coke.

Mechanism of coke formation
The process of thermal refraction with hydrocarbon vapor (pyrolysis) is a highly endothermic reaction and the necessary energy is provided by a furnace. This process consists of a series of radical reactions in which the complexity of the reaction mechanism depends on the nature of the feed.
During favorable reactions, adverse reactions are also formed, which causes coke in the furnace coils (reactor). Coke formation is an acute problem and limits the operation time of the furnace and leads to a temporary loss of production capacity and coking operations.
The main sources of coke formation are substances such as Olefins, benzene, toluene, xylene and styrene. In addition to reducing production capacity, coke formation leads to pressure drop, corrosion, hot spots and carburization of furnace coils. Reducing the rate of coke formation requires proper knowledge of the pyrolysis process, coke formation and proper selection of inhibitory chemicals.
Coke formation can occur through the following three mechanisms:
• Coke formation by catalytic mechanism
• Coke formation by asymptotic coking mechanism
• Coke formation due to condensation of polyaromatic compounds
They are widely used in Olefin kilns to achieve the following purposes:
• Increase the speed of thermal cracking
• Selectivity of hydrocarbon conversion
• Process the inner surface of the reactor to prevent coke formation
According to studies, the following sulfur compounds are used to prevent coke formation in Olefin processes.
• Elemental sulfur
• Hydrogen sulfide (H2S)
• Dimethyl sulfide (DMS)
• Dimethyl disulfide (DMDS)
• Thiophane
• Benzyl sulfide
• Benzyl disulfide
Coke also settles in the reactor wall as an unwanted product and can affect the operating conditions and efficiency of the furnace. The efficiency of manufactured products in heat failure reactors depends on several operational parameters, including the following:
• Composition in the feed
• The amount of feed
• Temperature
Coke is one of the undesirable by-products produced in the thermal failure process. Coke is composed of polycyclic aromatic compounds and settles as a carbon-rich solid deposit in the reactor wall. Adverse effects of coke build-up on reactor walls include:
• Reduce heat transfer
• Increased pressure drop in the reactor
• Increase fuel consumption
• Increase the reactor wall temperature
• Carbonization of reactor pipes
• Reduce reactor life
The heavier the food, the more coke and aromatic substances are produced. Increasing the temperature also leads to more coke production. For this reason, the highest amount of coke is formed at the reactor outlet, which has a higher temperature. The rate of coke formation depends on the characteristics of the feed, including:
• Molecular Weight
• The amount of sulfur in food
• Analysis (Paraffins, Iso paraffins, Naphthenes, Aromatics)
The rate of coke formation increases with the change of feed analysis from paraffin to naphthene and from naphtha to aromatic hydrocarbons. Aromatics with a structure similar to the structure of coke are one of the most important sources of coke formation.
Figure below shows simple scheme mechanism of coke production in low temperature.

Simple scheme mechanism of coke production in low temperature

simple scheme mechanism of coke coating

simple scheme mechanism of coke coating
Predicting the mechanism of coke formation using modeling
Making changes during the operation of the Olefin unit in order to study how the unit operates and responds to changes applied, due to the high volume of production of this unit and the long duration of stabilization of the conditions that result in total waste of materials is very costly.
Therefore, in order to reduce the response time and reduce the incidental costs of changes that can reduce the unit life, the modeling and simulation of the furnace is discussed. In the modeling section, based on the study of furnace conditions and the type of operation that takes place, the required equations are extracted. These equations are obtained by modeling the balance of the following equations:
• Mass transfer Equation
• The heat transfer Equation
• Momentum Equation
All equations are based on basic assumptions that have the task of both simplifying the equations and defining the mathematical relations of the equations of mass transfer of mass, heat and momentum.
In the mass kinetic equilibrium, reactions are considered based on molecular and radical (fundamental) models, in which a total of 32 main material species are linked by 64 molecular reactions. In the subject of simulation, the sum of the equations obtained is solved by different methods.
Industrial furnace data is used to apply the required mathematical boundary conditions. The output of the program is a variety of temperature and concentration profiles that are used after checking the degree of compliance with the information of the industrial unit in order to predict other cases and operating conditions. Among the applications of this model after validation, the following can be mentioned:
• Coke formation rate
• The effect of operating conditions on the rate of coke formation
• Calculate coke thickness
• Check for clogging inside the reactor pipes
• Optimization of unit operating conditions
Effects of coke formation on chemical processes
Coke formation in the Olefin process, as a by-product, can have significant adverse technological effects on operational processes. Coke can form both inside the pyrolysis coils and the quench (TLE) system.
Take. This phenomenon causes the following:
• Increased pressure drop in the coils
• Hot Spot Phenomenon
• Coil corrosion phenomenon (carbonization)
• Reduce the life of the reactor
• Reduce run time
• Decreased product production
• Reduce product selectivity
• Increase in repair costs
• Increase energy consumption
It is clear that under the conditions of heat failure, the formation of coke is inevitable and can not be completely prevented, but practical approaches to reduce or moderate the formation of coke have been done by industry so far and some are being done, all based on Reduction of coke formation in coils and the amount of separation
Coking or coking agents during cracking are as follows:
• Upgrading the metallurgy of coils from HP and MOD to 35Cr / 45Ni
• Add biodegradable sulfur to the feed at cracking temperature
• Use of anti-foulant scale materials
• Coke gas conversion to hydrogen and carbon monoxide in the vicinity of the catalyst
• Covering and reinforcing surfaces using Mg, Si, Al and Cr (Special Coating)
• Increase the amount of diluent vapor relative to feed