Methanol (2)
As mentioned in the previous section, modern industrial-scale methanol production today is performed from a high-pressure mixture of hydrogen gases and carbon oxides on a metal catalyst. The pressure of the synthesized gas depends on the activity of the catalyst used. Methanol production technologies are classified into three groups:
- Low pressure processes (5 – 10 MPa)
- Medium pressure processes (10 – 25 MPa(
- High pressure processes (25 – 35 MPa)
Figure (1) shows two different mechanisms of methanol production.
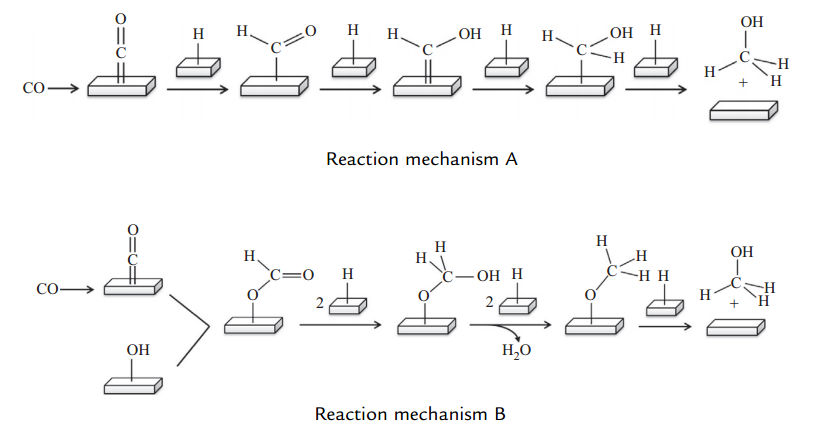
Fig (1): Two different mechanisms of methanol production.
Applications of methanol
Methanol usually participates in reactions that are chemically in the category of alcoholic reactions. Important industrial applications of methanol include dehydrogenation, oxidation of methanol and conversion of aldehyde on silver or molybdenum-iron catalysts, as well as conversion of methanol to acetic acid on cobalt or rubidium catalysts.
On the other hand, dimethyl ether (DME) can be produced by acidic catalyst using methanol dehydration. The reaction of isobutylene with methanol in the presence of an acidic catalyst results in the production of methyl-tertiary-butyl-ether (gasoline octane booster). Methyl esters are produced by the reaction of carboxylic acids and methanol in the presence of an acidic catalyst.
The following substances are also produced by the reaction of methanol with the corresponding inorganic acids:
- Methyl hydrogen sulfate
- Methyl nitrate
- Methyl halides
The following chemicals are also obtained by direct reaction of ammonia with methanol:
- Mono-methylamine
- Di-methylamine
- Tri-methylamine
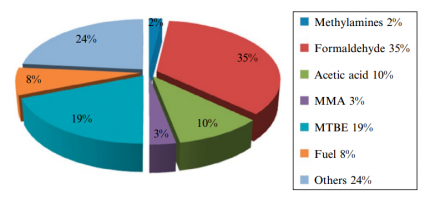
Figure (2) shows the production of different chemicals from methanol.
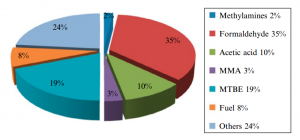
Fig (2): Production different products from methanol