Hydrogen Peroxide
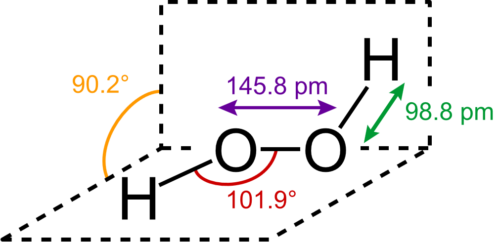
Hydrogen peroxide is a colorless, clear, slightly viscous liquid with a faint specific odor and can be mixed with water in any proportion. Hydrogen peroxide or hydrogen peroxide with the formula H2O2 is a weak acid. Oxygenated water is sold commercially in percentages of 35%, 50%, 60% and 70%. Oxygenated water, a dangerous non-combustible and explosive liquid, is a powerful oxidizing agent that reacts strongly with various substances. It can decompose spontaneously into water and oxygen and mix with water in any ratio. Oxygenated water is known as the simplest peroxide, a compound with a single oxygen-oxygen bond. The molecular structure of hydrogen peroxide is shown in Figure (1).

Figure (1): Molecular structure of oxygenated water
The process of producing oxygenated water
The most common oxygenated water production processes are:
- Oxidation process
- Electrolysis process
Oxidation process
In this process, one of the anthraquinone derivatives, such as 2-ethyl anthydroquinone, is converted to ethyl anthraquinone by reaction with hydrogen in the presence of a Pd catalyst. By passing air (O2) from the latter material, 20% by weight of hydrogen peroxide solution is obtained.
Electrolysis process
Electrolysis of 50% by weight sulfuric acid solution, or by electrolysis of sulfuric acid solution and ammonium hydrogen sulfate, with high current intensity, is obtained at the hydrogen peroxide anode and at the H2 cathode. In this reaction, a peroxysulfate ion is formed, from the hydrolysis of which H2O2 is obtained. The resulting hydrogen peroxide is rapidly separated by distillation at high temperature and low pressure, then the dilute solution of hydrogen peroxide is distilled at low pressure to obtain a 30% by weight solution of oxygenated water. Distillation can even increase the concentration of hydrogen peroxide to 99% by weight.
Properties of hydrogen peroxide
Hydrogen peroxide has valuable properties and significant properties that we will mention in the section on moist health:
- Effectiveness in the whole pH range
- High oxidative potential
- Its by-products do not cause pollution
- Ease of use (liquid)
Applications of oxygenated water in medicine
One of the most important uses of oxygenated water is its therapeutic properties. Oxygenated water is used in the following cases in this area:
- Wound cleansing and disinfection: Use on small wounds and cuts
- Sore throat
- Remove ear wax
- Teeth whitening
- Foot fungus
- Soften calluses and corns
- Relieves dry skin
- Get rid of mouth sores
- Treatment of ear infections
- Treatment of sinusitis
- Treatment of toothache
- Treatment of fungal infections
- Used as a deodorant
- Clean the lens of the eye