Citric Acid
Citric acid is an organic acid known by the molecular formula C6H8O7. The appearance of this compound is solid, crystalline and white powder. The acidic strength of this compound is high and this acid is divided into two types:
- Dry citric acid (anhydrosis)
- Hydrated citric acid (monohydrate)
Citric acidity is due to the presence of three carboxy groups that can lose protons. Citric acid ion is known as citrate. Citrates are good buffers for controlling the pH of acidic solutions. Citrate ion forms citrate with many metal salts, the most important of which is calcium citrate (lemon essence), which is used to keep food from spoiling and to make it taste good. Citrate ions can form chelating compounds with metals. Figure (1) shows the molecular structure of citric acid.
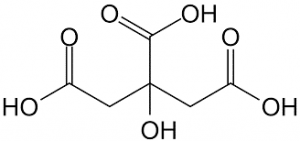
Fig (1): Molecular structure of citric acid
Citric acid is mainly used as a food preservative and food seasoning. It is widely used for cleaning and decriminalization of stainless steel boilers. The acid buffer solution is also used as a scavenger to remove sulfur dioxide from the gaseous fuels of power plants and refineries. Other applications include paints, oil extraction and metalworking water.
Citric acid production processes
Although citric acid can be found in the oral grade in high concentrations in many citrus fruits, it is not economical to extract it on a commercial or industrial scale. In addition, the current volume of demand for this substance is far from the amount that can be obtained from citrus. The following is a brief description of common citric acid production processes.
- Microbial fermentation
The discovery of citrate accumulation by Aspergillus niger led to the rapid development of the fermentation process, and today a large part of global citric acid production is done using this process. The most important characteristics of this process are the use of cheap raw materials and high efficiency.
- Chemical reactions
Citric acid was first synthesized by Grimaux and Adam in 1880 using glycerol as a chemical starting material, but this method was not economically competitive enough compared to other production routes such as fermentation.
- Production through natural
The method of extracting citric acid from lemon juice was done in 1784 by a Swedish chemist, Karl Wilhelm Shell (1742-1742). This method was adopted in England around 1826 for the commercial production of citric acid using lemons imported from Italy. This method retained its monopoly as the only commercial source for citric acid production until the late nineteenth century.
Applications of citric acid
- In the beverage and juice industry
- In the cosmetics and pharmaceutical industries
- In the food industry, in addition to flavoring, it also regulates the pH.
- In the production of detergent compounds