The Importance of Chemistry in Human Life
Chemistry is derived from the ancient Egyptian language and from the word khameh which means black earth. After Iran’s domination over Egypt, the word alchemy made its way to the East. In 330 BC, it entered Greece under the name of Khomeia (χυμεία), and with the beginning of the Islamic rule in the Middle East, this word was changed to alchemy, and with the Crusades, it was called alchemy. Chemicals are not only not harmful, but they can even play a special role in the structure of the human body, contrary to the idea formed in most of the public minds.
If it wasn’t for chemistry, maybe there would never be tears on our cheeks and there would never be a feeling of hunger. This amazing science studies the composition, structure and properties of matter and its interaction with other substances and energy, and examines the changes that different forms of matter undergo in various conditions.
In this article, the uses of chemicals in different fields are described. Chemicals are the building blocks and necessary for everything. All living things are made of chemicals. Accordingly, chemical compounds are used in the industry to advance the processes. These substances are found in many consumer goods.
Chemistry is the scientific study of the properties and behavior of matter and examines the constituent elements of matter, compounds consisting of atoms, molecules and ions, structure, properties, behavior and the changes they undergo during the reaction with other substances. Also, the interaction between atoms and molecules with chemical bonds and the formation of new compounds is investigated and carried out by chemistry. All available compounds have at least one of the following links:
- Ionic
- Van der Waals
- Covalence
- Hydrogen
Modern chemistry includes principles, the most important of which are Matter in chemistry, matter is anything that has mass and volume at rest. Particles that make up matter also have rest mass. There are particles like photons that have no rest mass.
Atom
The atom is the basic unit of chemistry. It consists of a dense nucleus called the atomic nucleus, which is surrounded by a space occupied by a cloud of electrons. The nucleus consists of positively charged protons and uncharged neutrons.
Element
A chemical element is a pure substance that consists of one type of atom and is determined by a certain number of protons in the nucleus of its atoms, which is called the atomic number and is denoted by the symbol Z.
Composition
The composition of a pure chemical substance is composed of more than one element. Also, the properties of compounds have little resemblance to the properties of constituent elements.
Molecule
A molecule is the smallest indivisible part of a pure chemical substance and has a unique set of chemical properties.
Phase
A phase is a collection of states of a chemical system that have similar bulk structural properties over a range of conditions, such as pressure or temperature. The most familiar examples of phases are solids, liquids and gases.
All our daily activities such as drinking water, showering, cooking, cleaning the car, laughing or even crying are driven by various chemical processes in the body.
In the following, the properties and use of chemicals in daily life have been examined:
Body composition
Our body is made up of various chemical compounds, the most important of which is water, the substance that covers most of our body and is the result of the chemical combination of hydrogen and oxygen. The function of chemical transmitters, especially nerve messengers, is also due to their chemical nature. Chemical substances such as the following are responsible for these processes in the body, and all of their functions can be justified by chemistry:
- Serotonin
- Epinephrine
- Dopamine
The human body is made up of various elements. The two main components of the human body are:
- Carbon
- Oxygen
In addition to the above elements, there are other elements in our body, which are:
- Nitrogen
- Phosphorus
- Hydrogen
- Calcium
- Potassium
- Sulphur
- Magnesium
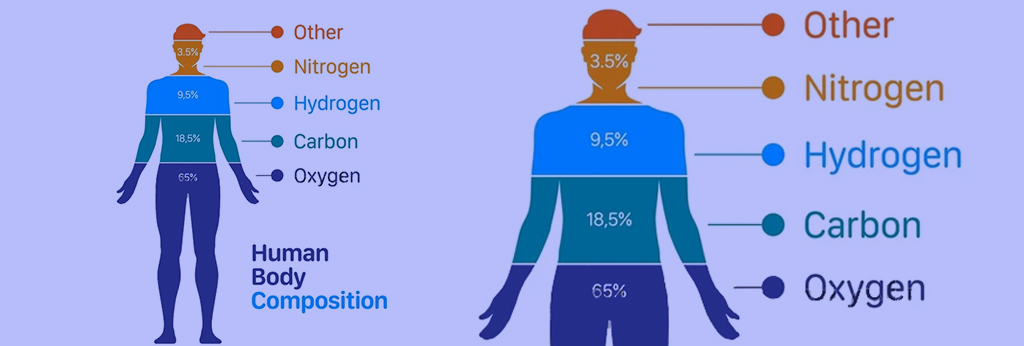
The lack of any of these elements can cause various complications for the body, which is usually compensated to some extent with the help of supplements. Figure (1) shows the percentage composition of the elements that make up the human body.

Fig (1): The composition of the percentage of elements that make up the human body
You can see the names of these elements and the basic role of each in the body as follows:
- Oxygen (primary solvent and regulation of temperature and osmotic pressure)
- Carbon (source of energy, builder of body cells)
- Calcium (an essential element for bones and muscle contraction)
- Manganese (this substance helps in fat and carbohydrate metabolism, calcium absorption and blood sugar regulation, building bones and blood clotting factors.)
- Magnesium (required in more than 300 biochemical reactions)
The presence of chemical elements and their reactions are not only limited to the presence in the human body, but these elements also play an important role in the emergence of emotions. Whenever we feel happiness, sadness, joy, peace or stress, many chemical reactions take place in the body. In these cases, chemical messengers or neurotransmitters are released in the brain.
From the moment you put food in your mouth, a number of different chemical reactions begin in your digestive system. Saliva contains the enzyme amylase, which is responsible for breaking down carbohydrates, and the stomach begins to produce hydrochloric acid. All these enzymes undergo chemical reactions for proper digestion and assimilation of food.
Food Production
Plants produce their food through photosynthesis, which is a very important chemical reaction. The chemical reaction that occurs in photosynthesis is the most common and most vital chemical reaction.
Chemicals are present even in the best and tastiest natural fruits. For example, there are more than 350 chemicals in strawberries, the most important of which are:
- Methyl butanoate
- Anthocyanins
- Auxin
If you read the ingredients on the label of products such as ketchup, jam or pickles, you may be surprised to see the list of different chemicals. Most of these chemicals are known as food preservatives. These preservatives delay the growth of microorganisms. The most common chemical food preservatives are:
- Sodium benzoate
- Sorbic acid
- Potassium sorbate
- Calcium sorbate
- Sodium lead
- Propionic acid
- Nitrous acid salts

Sanitary materials
Soaps are sodium or potassium salts of fatty acids that are formed during the chemical processes of saponification. Soaps react with grease and oils and can remove them from surfaces. Among the chemicals used in soaps, the following compounds can be mentioned:
- Sodium hydroxide
- Potassium hydroxide
Bread, cake and sweets
Good bread is the result of chemical reactions. The main components of bread are yeast, flour, water and salt. Each of these components plays a role in baking bread. Flour contains starch and protein. Flour and water are mixed with yeast to make dough. In the mixed dough, water and protein create chains of complex molecules called gluten. When the dough is kneaded, these chains line up and the dough becomes smooth and uniform. The starch in the dough turns into a jelly-like substance with water and gives a special structure to the dough.
What changes occur in food during cooking, how food spoils, how food can be stored, and how the body works to digest and absorb it, all these things depend on the science of chemistry and reactions. chemically related.
In order to improve the quality and texture of bread, cakes and sweets that are consumed, baking soda additive with the chemical name of sodium bicarbonate is used. Adding baking soda to food before cooking leads to the production of carbon dioxide (CO2).

Sunscreens
Sunscreens are among the materials that are used abundantly especially in summers. Zinc oxide and titanium dioxide are among the chemicals that are generally used in these products. These substances prevent the penetration of ultraviolet rays deep into the skin.
Iron rust
Over time, orange and brown coatings form on iron products. These coatings are called iron rust. Rusting of iron is a type of oxidation reaction. The atoms in iron metal undergo oxidation and reduction. The chemical reactions that occur during this process are as follows:
Fe + O2 + H2O → Fe2O3. XH2O
However, there are many anti-corrosion and anti-rust compounds that prevent this process from occurring. Among these things, the following compounds can be mentioned:
- Benzotriazole
- Tolyltriazole

Batteries
As societies become more industrialized and more people use mobile phones and electric cars, the role of batteries in everyday life becomes more visible. Lithium batteries and the use of cobalt sulfate in order to increase the thermal resistance of batteries has been able to predict an optimistic market for batteries.
Medicine and pharmaceuticals
The science of chemistry and chemical compounds has helped a lot to improve health and treatment facilities and saved many lives. Since the past, many doctors have taken help from chemistry to diagnose chemical reactions in the human body, and it is possible to diagnose various diseases by performing tests such as blood tests with the addition of certain chemicals. Chemistry has played an essential role in making various medicines, including the following:
- Analgesic drugs to reduce various types of pain
- Antibiotics to control infections
- Tranquilizers to reduce stress and mental tension
- Antiseptics to prevent infection of wounds
- Anesthetic drugs for ease of performing surgeries
- Pesticides that have greatly reduced the risk of diseases caused by mice, mosquitoes and flies
Application of chemistry in industry
Chemistry has played an important and key role in the development and growth of a large number of industries, and chemical reactions and chemicals are used in the manufacture of almost all products that are produced in factories. The unique applications of chemistry in industries such as oil, glass, cement, paper, fabric, paint, plastic, pharmaceutical, etc. are undeniable, and without a doubt, all these materials are considered to be one of the most important needs of humans today.
In addition to this, throughout history, chemistry has helped to produce more and more sulfuric acid, nitric acid and ammonia, which are widely used in industries, by providing suitable catalysts.
Application of chemistry in agriculture
Farmers use various chemical fertilizers to increase the quality of their fruits, vegetables and other crops, among which the following fertilizers can be mentioned:
- Urea fertilizer
- Calcium superphosphate
- Sodium nitrate
- Ammonium sulfate
In addition to chemical fertilizers, chemical products are also used in agriculture for the following purposes:
- Insecticides: one of the types of pesticides used in agriculture to kill insects and pests
- Fungicides: in order to destroy and prevent the growth of fungi
- Antibacterials: in order to destroy various bacteria and viruses
- Anti-rodent: to eliminate rodents such as mice
- Herbicides: in order to destroy unwanted weeds
Application of chemistry in the environment
Thanks to scientific advances, there are now many chemicals that are environmentally friendly and help us preserve nature and the environment around us. The science of chemistry has come to our aid in cleaning the environment and we can prepare the materials we need with some chemical methods in such a way that the damage to the environment is minimized.
Application of chemistry in legal issues
Chemistry plays an important role in the field of law and examining legal cases. For example, a person mixes kerosene with gasoline and sells it, to prove this issue in court, chemical tests are used to confirm or deny the accusation of the person in question.
Other common applications of chemistry in daily life
With the progress of chemistry and thanks to numerous chemical discoveries, today’s life has become easier than in the past. The applications of chemistry in our daily life are beyond imagination and chemical compounds have been widely present in the production of many of our essentials and conveniences, including:
Artificial fiber
Synthetic fibers such as nylon, and rayon are attractive, comfortable, and durable fibers that are easy to wash, dry quickly, and do not require ironing.
Variety of colors
With the production of chemical and synthetic dyes, our world has become more colorful and this issue has had a significant impact on the variety of clothing and accessories around us.
Building Materials
Chemical science has been used in the production of useful materials such as steel and cement, and the result has been the construction of safer houses, stronger dams, and more durable bridges.
Mining and production of metals
Chemicals are used in the extraction of various metals, including the metals used for this purpose, including:
- Gold
- Silver
- Copper
- Iron
- Aluminium
- Zinc
In addition to the above, many alloys of these metals are also produced with the help of chemistry, which are widely used in the manufacture of various objects, including ornaments, dishes, coins, and many industrial and agricultural supplies.

The use of chemicals in the printing industry
If we want to introduce all the chemicals used in the printing industry, a wide range of elements of the Mendeleev table will be included, but we will refer to the most common and widely used chemicals used in the printing industry. In printing inks, we see the use of xylene, toluene, and xylene in a wide way, and zinc sulfur is used to make the colors used. It may be interesting to know that alcohol plays a significant role in the printing industry. In this industry, alcohol is used. They are used as a solvent for the construction of printing compounds. Ethanol, normal propanol, methylcyclohexanol, hexylene glycol and dipropylene glycol are widely used in the field of printing.
The use of chemicals in the detergent industry
Separation of chemicals from the detergent industry is not possible in any way, and if there are no chemicals, the survival of this industry will not be possible. Detergents appear as saviors to clean and remove any stains, dirt and grease from all surfaces. It may not be an exaggeration to introduce soap as the first human-made detergent. The use of chemicals such as glycerin, fatty acids and sodium salt in the soap production process cannot be denied. Surface modifiers, water and additives are used in making shampoos. Disodium laureth sulfosuccinate is used in shampoos to clean pollution.
Disodium laureth sulfosuccinate is used in shampoos to clean pollution. The chemicals used in shampoos are polysorbate 20, polyethylene glycol 150 and diethanolamine fatty acid coconut. Citric acid is also used to adjust the pH of the scalp and Anti-dandruff properties can be found in the chemical composition of shampoos. Detergents are products that contain an active substance called surfactant or surface active substance aluminum hydroxide. Surfactants can lower the surface tension of water so that water mixes with oil or fat. This is why we wash dirty clothes with detergents. Detergents can remove contaminants in solid or liquid form.
Detergents in shampoos can also reduce the surface tension of water and wash off grease and dirt from the hair by wetting the hair. You can see a long list of chemical compounds used in these products on their labels that are able to remove dandruff, grease and dirt from your hair. Toothpaste is one of the other health care products that we all use regularly. We use it frequently. Toothpaste is actually a chemical compound that is made of water, carboxymethyl cellulose and abrasive materials such as aluminum hydroxide and calcium carbonate. This product also contains sweeteners, colors, breath fresheners, and disinfectants. There are microbes and an active substance called sodium fluoride that strengthens tooth enamel and protects against decay.
The application of chemical in Computer technology
A microchip is one of the main components of a computer, which is made of silicon, which is a type of polymer, and plastic is also used in the construction of the external parts of the computer. Both of these materials are the result of chemical processes that have been carried out in factories to produce them.
The use of chemicals in Archaeology
Measuring the age of fossils is done using radioactive isotopes and with the help of chemical reactions to clean the dust on them without damaging the historical monuments.
Cars are the main source of raw materials that produce smog. Nitrogen oxides, especially nitrogen dioxide and hydrocarbon fumes, mix with air and other pollutants. This mixture produces ozone, other nitrogen oxides and sulfur oxides due to sunlight.
Over the years, chemistry has been used to control vehicle fumes, including:
- Modification of engines
- Catalytic converters
- Afterburners as a supplement to the burning of waste gases
Chemicals play a significant role in our economy. Therefore, the correct management of these substances during their life cycle (from extraction, production to disposal) is necessary to prevent endangering human health and the environment. Chemicals have many benefits in our lives. But at the same time, we should treat them carefully to minimize the harmful effects of using chemicals. For more information, you can refer to the article related to the types of chemicals on the site.

