Calcium hypochlorite
Calcium hypochlorite is a chemical with the formula Ca(ClO)2. Calcium hypochlorite is a white substance with disinfectant properties that disinfects water, vegetables, fruits, dishes, baths, toilets and Infected areas are used. Calcium hypochlorite is a strong oxidant and safety considerations should be considered when working with it. The chlorine atoms in this compound cause its disinfecting and disinfecting properties.
When Calcium hypochlorite is exposed to microbes and other organic contaminants, the chlorine compounds in it disrupt the function of their cells by binding to enzymes and other cell components. This inactivates the bacteria by inactivating its internal work or by disrupting the cell walls by disinfecting. Figure (1) shows the molecular structure of calcium hyperchlorite.
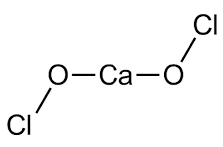
Figure (1): Molecular structure of Calcium hypochlorite
Calcium hypochlorite is prepared by reacting chlorine with lime in the presence of sodium hydroxide. In a multi-step process, Calcium hypochlorite slurry is prepared, which is produced by filtration and drying to obtain granules of the final product. The final product is usually offered in two ways:
- White chlorine powder (purity 39% -33.5%)
- Granules (purity 70 %- 65%)
The final product is in the form of granules and is offered in plastic barrels of 40, 45 and 50 kg.
Due to its sensitivity to heat and humidity, calcium hypochlorite should be stored in a cool, dry place, and storage in the sun or in hot storage poses a risk of explosion. Also, Calcium hypochlorite barrels should not be placed near odorous and flammable materials such as gasoline, oil, diesel or agricultural pesticides, especially phosphorus. The storage tank for Calcium hypochlorite barrels must be properly ventilated and the floor of the storage tank must be made of moisture-resistant material. Calcium hypochlorite barrels should be placed on wooden plates 10 cm from the floor of the warehouse.
Calcium hypochlorite is relatively stable and is similar to sodium hypochlorite in its antiseptic properties and has more chlorine present than sodium hypochlorite. This nocturnal compound has excellent storage stability and its strength is maintained over time. Applications of Calcium hypochlorite include:
- For drinking water treatment
- bleaching reagent (paper, textiles)
- Antifungal, antibacterial (odorless)
- Disinfect and disinfect pool water

