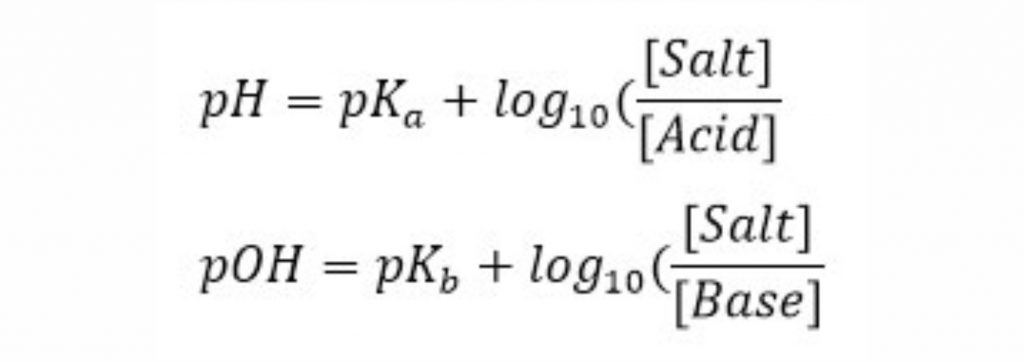Buffer solution
H+ ion is an acidic agent and OH– ion is a base or base agent. A neutral solution means that the number of two ions in a solution is the same and the solution is in equilibrium. If for any reason the amount of these two ions in the solution changes, the solution changes in terms of acidity or alkalinity.
Practically, to determine the acidity or alkalinity of a solution, the amount of H+ ion in the solution is measured as pH. The pH of a solution varies from 0 to 14. The pH is between 0 and 7 acidic and 7 to 14 base, with a pH of 7 indicating the neutrality of the solution. Figure (1) shows the range of pH changes for acidic, neutral, and base solutions.
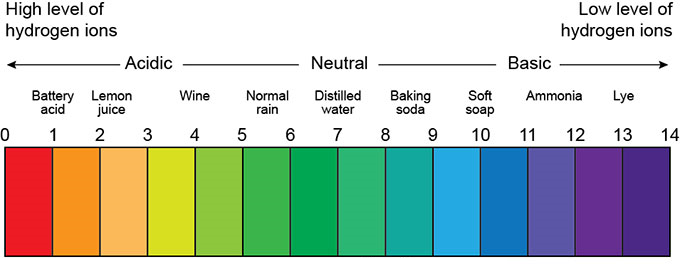
Fig (1): The range of pH changes for acidic, neutral, and base solutions
Buffer solution is a blue liquid chemical solution that has other names such as hydrogen ion buffer. This solution is made from a combination of very weak acidic material and also very weak base with its salt, so it can be said that this material can have both acidic and base properties and cannot be used in the base and Or acids are contracted. For this reason, the properties of this solution include the ability to neutralize acids and also to neutralize bases.
Types of buffer solutions
There are two types of buffers:
- Acid buffer
- Base buffer
An acidic buffer is formed from a buffer solution containing several amounts of weak acid and its salt with a strong base, hence the name acidic buffer. Such buffer solutions have an acidic pH of less than 7.
In the definition, play buffer is said to consist of a buffer solution containing relatively large amounts of weak base and its salt with a strong acid, and therefore its name is play buffer solution. The pH of such base buffers is more than 7.
To calculate the pH of an acid-base buffer solution from an equation first proposed by two chemists, Lawrence Joseph Henderson and Carl Albert Hasselbalkh. This equation is defined as follows:
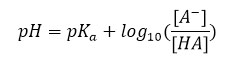
In this relation [HA] is the weak molar concentration of insoluble acid, [A–] is the conjugate base concentration of this acid and is where the dissociation constant is acidic. Using the above equation, the pH of acidic and base solutions can be obtained as follows:
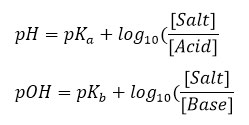
In these relationships, and are weakly acidic acid content and weakly basic base dissociation content, respectively.
Industrially, buffer solutions are used in the following processes:
- Fermentation processes
- Colored fibers
- In chemical analysis and pH calibration of meters
- Natural and industrial leather production industry
- Preparation of photographic materials