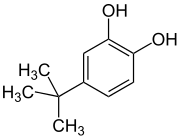
4-tert-butylcatechol 98% (TBC) (production process)
The theory currently proposed for the decomposition of organic matter is based on the formation of hydrocarbon radicals. Decomposition of materials occurs due to physical or chemical factors such as heat, light and mechanical energy.
The free radical formed reacts with oxygen in the air and is converted to the peroxide radical, which then combines with a large number of hydrocarbon molecules to form the primary hydrocarbon radical and the peroxy compound. Finally, the peroxyde compound is decomposed into aldehydes, ketones and carboxylic acids and, depending on the destructive action, is responsible for the staining, corrosion and unpleasant odor of the organic compounds.
The above chain reaction is stopped by the free radicals combining with each other. However, the probability of such a reaction being stopped is very weak, so that the radical decomposition of organic compounds does not stop without the addition of other substances. These materials, which contain monovalent or divalent phenols with large branching groups, act as adsorbents. These compounds bind to free radicals to prevent the release of chain reactions.
The antioxidant role of phenolic compounds depends on the two effects of space and induction, which manifests itself in the molecular structure of this type of compounds. For example, the inherent stability of alkylated phenoxy radicals is due to its resonant properties as well as the bulky Tertiary group in the ortho or para position, which increases its antioxidant power. Phenoxy radicals can be stabilized by hydrogen bonding. For example, the proximity of two hydroxy or amino groups in the benzene ring found in catechols or catechol amines increases radical stability.
There are various methods for making this substance, which can be referred to the reaction of butyric alcohol catechol in the presence of 85% phosphoric acid and xylene. Instead of phosphoric acid, 66% sulfuric acid can be used, but the yield of the reaction is reduced. In another method, in the presence of ion exchange resins as a catalyst and xylene as a solvent, catechol reacts with isobutanel to produce TBC.
In another method, 4-and 6-di-tertiary butyl-3-methylphenol reacts with catechol in the presence of perchloride and produces TBC. Another method for the synthesis of TBC is the reaction of catechol with methyl tertiary butyl ether in the presence of an acid catalyst which is separated from the alcohol component by distillation.
The TBC method consists of a component such as catechol and a gaseous reactant such as isobutylene gas in the presence of an acidic catalyst such as sulfuric acid or Lewis acid such as AlCl3, BF3, HF and H3PO4. This reaction is of the Friedel Craft type and is based on the formation of the third type of carbation. The formed ion attacks the aromatic ring and the product is formed.
Non-mineral catalysts are used in the manufacture of this material, which has more advantages than mineral-based acid catalysts in various ways. This catalyst is an ion exchange resin and is used in many reactions and has many applications in the petroleum and petrochemical industries. Such as benzoyl peroxide and then sulfonation of the reaction mixture.